Interface properties studied by the use of various probe molecules
Interface properties studied by the use of various probe molecules
Solvation at interfaces was investigated by heterodyne-detected electronic sum frequency generation (HD-ESFG). It was found that each solvatochromic probe molecule feels a different effective polarity at the air/water interface, depending on its orientation at the interface. It was also found that the air/water interface provides a more inhomogeneous solvation environment than bulk solvents. Further, such solvatochromic measurements at the air/water interface revealed that the pH of the air/water interface is slightly more acidic than that in the bulk phase.
(Yamaguchi, S. Sen, P. Sen, Watanabe, S. Mondal, Kundu)
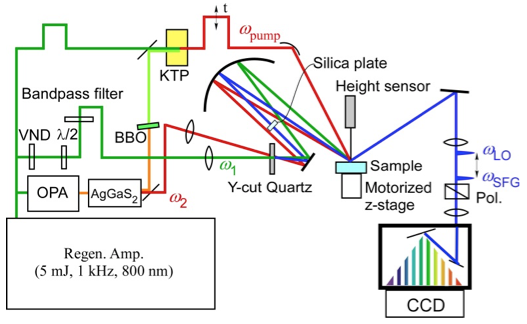
Fig. 1. Schematic of time-resolved heterodyne-detected vibrational sum frequency generation spectrometer.
